Assistant Professor Shunsuke Matsumoto and his research group have elucidated the mechanism by which proteins are precisely targeted to peroxisomes.
Retargeting proteins to ensure accurate delivery to specific subcellular destination
Points
- To ensure proper cellular function, it is essential to understand how proteins destined for organelles are accurately delivered to their appropriate subcellular locations.
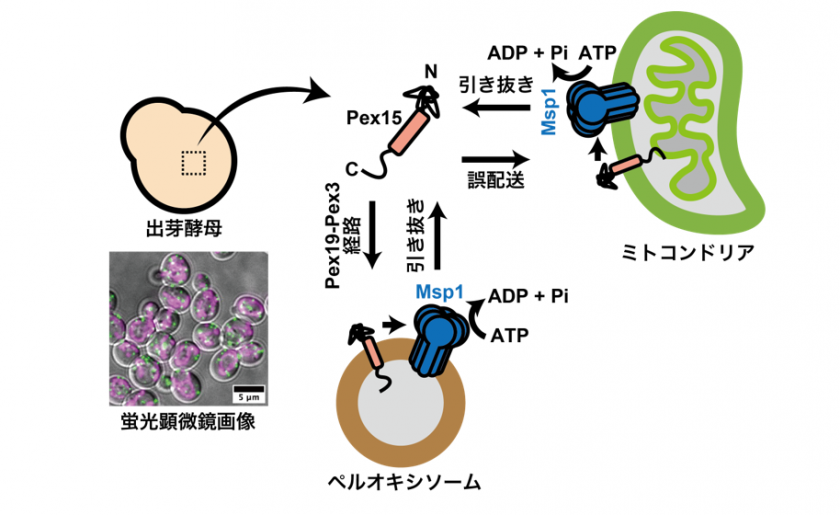
- The study revealed that Pex15, a membrane protein on peroxisomes*, can be retargeted to peroxisomes even after being mistargeted to mitochondria or extracted from the peroxisomal membrane.
- These findings are expected to provide insights into the fundamental principles of protein retargeting and may contribute to the development of therapeutic strategies for diseases associated with protein mislocalization.
Abstract
In eukaryotic cells, such as those of humans, the process by which proteins are delivered to intracellular organelles has long been considered highly precise, with mislocalized proteins thought to be rapidly degraded. However, recent our studies have demonstrated that errors in protein targeting can indeed occur, and that cells possess an intrinsic "proofreading" system capable of correcting such mistargeting events. Despite these findings, it remains unclear to what extent this "retargeting" mechanism is generalizable across different organelles and protein types.
A collaborative research group comprising Assistant Professor Shunsuke Matsumoto and Associate Professor Tomoyuki Numata from the Faculty of Agriculture at Kyushu University, Yoshiki Kogure, a second-year master's student from the Graduate School of Bioresource and Bioenvironmental Sciences, Professor Toshiya Endo and Researcher Suzuka Ono from the Faculty of Life Sciences at Kyoto Sangyo University, has demonstrated--through analyses using budding yeast--that the AAA-ATPase** Msp1, which localizes to both mitochondria and peroxisomes, together with the Pex19-Pex3 pathway, provides an opportunity for the retargeting of the peroxisomal membrane protein Pex15, thereby ensuring its correct localization.
Further elucidation of the protein retargeting mechanism is expected to deepen our understanding of disease pathogenesis and contribute to the development of novel therapeutic strategies.
This research was published in The FEBS Journal, an academic journal by John Wiley & Sons, on May 9, 2025.
Researcher's Comment
Through analyses in budding yeast, we demonstrated that Msp1 facilitates accurate organelle targeting by extracting mislocalized proteins from membranes and redirecting them to their original trafficking pathways. The MSP1 gene is highly conserved from yeast to humans, and future studies will focus on elucidating the retargeting mechanism in human cells. (Shunsuke Matsumoto)
Glossary
* Peroxisome
Peroxisomes are organelles found in eukaryotic cells, primarily involved in the breakdown of fatty acids and hydrogen peroxide, as well as in the detoxification of harmful substances such as alcohols and phenols.
** AAA-ATPase
AAA (ATPases Associated with diverse cellular Activities) -ATPases are motor proteins that hydrolyze ATP to generate energy, driving a wide range of cellular functions. Many AAA-ATPases form homohexameric ring structures with a central pore that serves as a channel through which substrate proteins or DNA are translocated. This translocation enables substrate unfolding, degradation, or extraction from membranes.
Paper Information
Journal: The FEBS Journal
Title: Msp1 and Pex19-Pex3 cooperate to achieve correct localization of Pex15 to peroxisomes
Authors: Shunsuke Matsumoto, Yoshiki Kogure, Suzuka Ono, Tomoyuki Numata, Toshiya Endo
DOI:10.1111/febs.70132
For Research-related inquiries










 Contact
Contact
 Access Map
Access Map

