Professor KAKUTA Yoshimitsu and Assistant Professor TERAMOTO Takamasa, along with their research group, have elucidated the three-dimensional structure of the enzyme GfsA.
-Expectations for the development of antifungal drugs and pesticides with novel mechanisms of action-
POINT
- Elucidation of the crystal structure of the galactofuranose transferase GfsA for the first time in the world
- Elucidation of the specific mechanism of galactofuranose transfer to the 5-hydroxyl group by GfsA
- Providing essential foundational insights for the development of new antifungal drugs and pesticides
SUMMARY
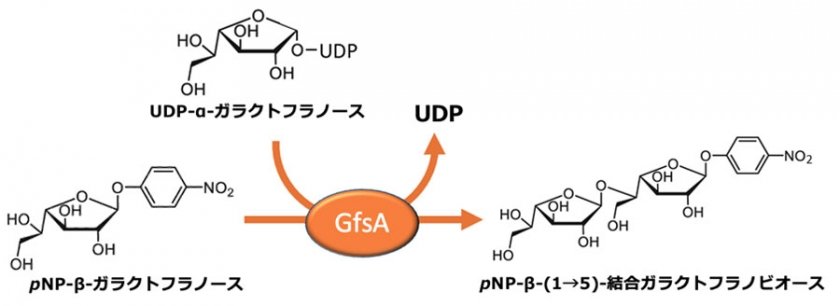
Aspergillus fumigatus is a major causative agent of pulmonary aspergillosis and can cause fatal infections, particularly in individuals with weakened immune systems. However, effective treatments for pulmonary aspergillosis are limited, and the emergence of resistant strains to existing antifungal drugs has become a global issue. The cell wall of fungi is an essential structure for protecting the cell, primarily composed of five types of sugar chains intricately intertwined in a three-dimensional manner. GfsA is a central enzyme responsible for the synthesis of galactofuranose chains, which are one of the sugar chains that constitute the cell wall. GfsA, discovered by Professor Oka and his team in 2013, is a glycosyltransferase that uses UDP-α-galactofuranose as a sugar donor. It continuously transfers β-galactofuranose to the 5-hydroxyl group of β-galactofuranose, synthesizing β-(1→5)-linked galactofuranose chains with a maximum chain length of seven (Figure 1). GfsA is the enzyme responsible for this reaction and has been assigned the EC number 2.4.1.398 by the International Union of Biochemistry and Molecular Biology. In strains where the gfsA gene has been disrupted, it has been confirmed that they are unable to form a normal cell wall, resulting in inhibited hyphal elongation.However, the crystal structure of GfsA and the mechanism of galactofuranose transfer reactions have remained unknown, posing a barrier to the development of antifungal drugs.
Professor OKA Takuji, Professor HIRA Daisuke, and Assistant Professor KADOOKA Chihiro from the Department of Biological Sciences, Faculty of Biological Sciences, Sojo University, along with Professor KAKUTA Yoshimitsu and Assistant Professor TERAMOTO Takamasa from the Faculty of Agriculture, Kyushu University, have elucidated the three-dimensional structure of GfsA, an enzyme responsible for synthesizing the rare galactofuranose chains found in the fungal cell wall, and have clarified the specific mechanism of sugar transfer to the 5-hydroxyl group.
This research was published in PNAS Nexus on October 25, 2024.
Paper Information
Title: Substrate binding and catalytic mechanism of UDP-α-D-galactofuranose: β-galactofuranoside β-(1→5)-galactofuranosyltransferase GfsA
Authors: Takuji Oka, Ayana Okuno, Daisuke Hira, Takamasa Teramoto, Yuria Chihara, Rio Hirata, Chihiro Kadooka, Yoshimitsu Kakuta
Journal: PNAS Nexus
DOI: 10.1093/pnasnexus/pgae482
Research-related inquiries










 Contact
Contact
 Access Map
Access Map

