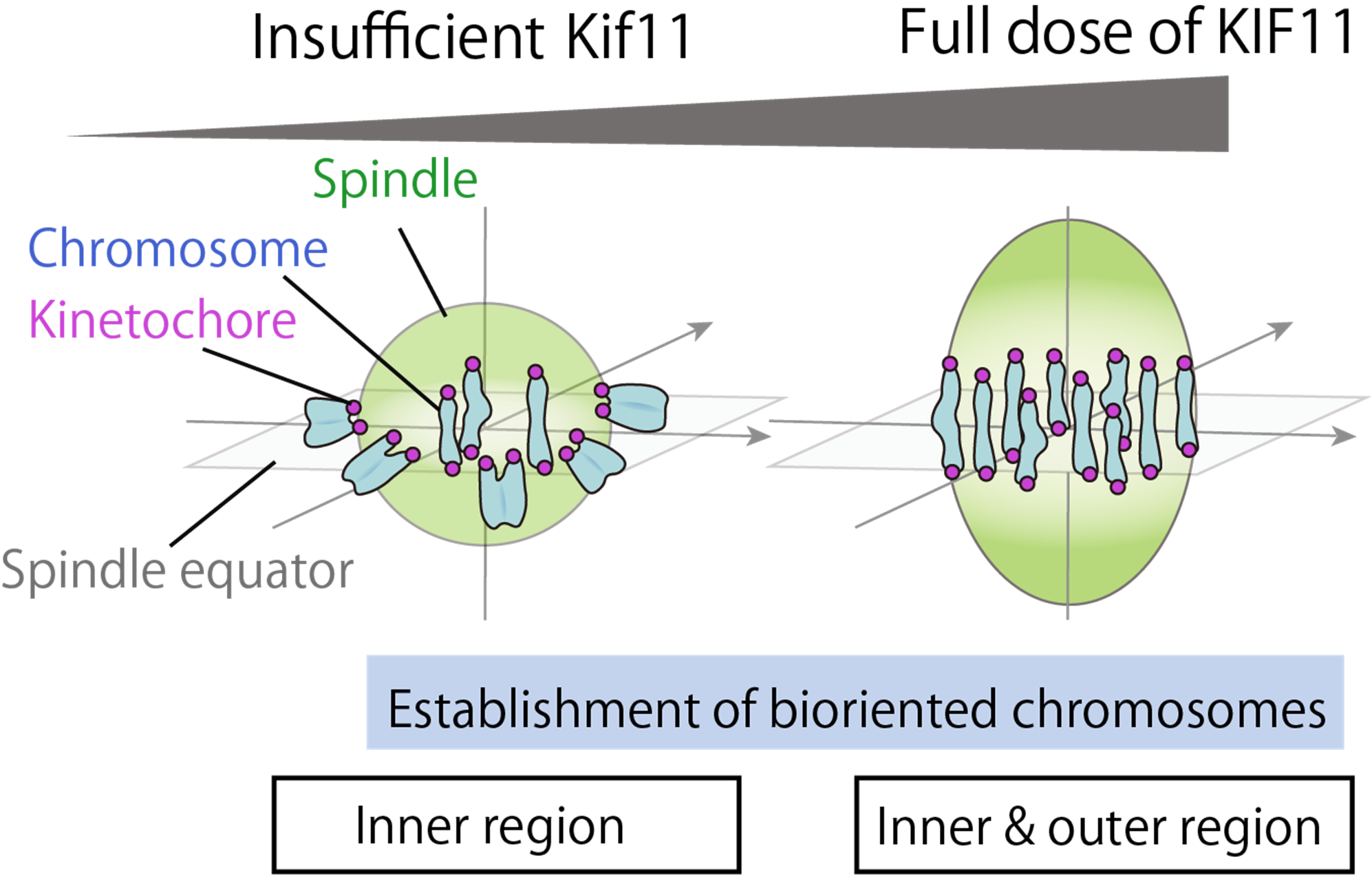
Assistant Professor Tappei Mishina and his research team have discovered that spatially different requirements for motors for chromosome biorientation in the oocyte spindle.
Revealing Spatially Differential Requirements Within the Spindle for Chromosome Biorientation During Meiotic Progression
Abstract
A collaborative research team led by Dr. Tomoya Kitajima, Team Director of the Laboratory for Chromosome Segregation at the RIKEN Center for Biosystems Dynamics Research, and Assistant Professor Tappei Mishina of the Faculty of Agriculture, Kyushu University (also a visiting researcher at RIKEN), has discovered that the required amount of the motor protein KIF11[1]-essential for accurate chromosome segregation during meiotic division[2] in oocytes-varies[3] depending on its spatial location within the spindle[4].
These findings are expected to provide new insights into the underlying causes of chromosomal abnormalities in oocytes and contribute to the development of improved diagnostic and therapeutic approaches for infertility.
Accurate chromosome segregation during cell division critically depends on spindle bipolarization and the establishment of biorientation, whereby microtubules[5] extending from both spindle poles attach to chromosomes from opposite directions. In this study, the collaborative research team employed a mouse model in which the expression level of KIF11-a motor protein essential for spindle bipolarization-can be genetically modulated. This enabled a detailed analysis of spindle formation and the establishment of chromosome biorientation in oocytes. The results revealed that when KIF11 expression is reduced to less than half, spindle elongation is impaired, particularly affecting chromosomes located at the periphery of the spindle, which fail to achieve proper biorientation. Furthermore, the study showed that erroneous kinetochore[6]-microtubule attachments are more frequent in the outer regions of the spindle, and that KIF11 plays a compensatory role in correcting these errors. These findings provide the first evidence of spatially differential requirements for KIF11 in the establishment of chromosome biorientation.
This study was published online in the scientific journal EMBO Reports on August 20 (JST).
Glossary
[1] Motor Protein KIF11
Motor proteins are a class of proteins that perform mechanical work within cells by utilizing chemical energy derived from the hydrolysis of adenosine triphosphate (ATP). KIF11 is a motor protein belonging to the kinesin superfamily (Kinesin Superfamily Proteins, KIF), and it plays a critical role in cell division by moving along microtubules, which are components of the cytoskeleton.
[2] Meiotic Division
Meiosis is a specialized form of cell division in eukaryotes that involves two successive divisions, resulting in the formation of gametes. It is essential for sexual reproduction and ensures the reduction of chromosome number by half. This study specifically focuses on the first meiotic division (meiosis I) in oocytes.
[3] Oocyte / Egg Cell
An oocyte is the precursor to an egg cell, the female gamete capable of being fertilized. Oocytes are formed during the fetal stage and remain in a prolonged dormant state until they resume development and mature into egg cells immediately prior to ovulation.
[4] Spindle
The spindle is a cellular structure formed during mitosis and meiosis to ensure accurate chromosome segregation. It is primarily composed of microtubules (see [5]) that organize and guide the movement of chromosomes toward opposite poles of the cell.
[5] Microtubules
Microtubules are long, cylindrical structures composed of polymerized tubulin proteins arranged in a ring-like configuration. They are key components of the cytoskeleton and play essential roles in maintaining cell shape, intracellular transport, and chromosome segregation during cell division.
[6] Kinetochore
The kinetochore is a protein complex formed on chromosomes that serves as the attachment site for microtubules during chromosome segregation. In mitosis, each sister chromatid develops its own kinetochore, referred to as sister kinetochores. In the first meiotic division, each homologous chromosome forms a single kinetochore, known as a homologous kinetochore.
Title: Kif11-haploinsufficient oocytes reveal spatially differential requirements for chromosome biorientation in the spindle
Authors: Tappei Mishina, Aurélien Courtois, Shuhei Yoshida, Kohei Asai, Hiroshi Kiyonari, Tomoya S. Kitajima
Journal: EMBO Reports
DOI:10.1038/s44319-025-00539-w
For Research-related inquiries










 Contact
Contact
 Access Map
Access Map

